Chemistry module under development: Things that (are) Matter
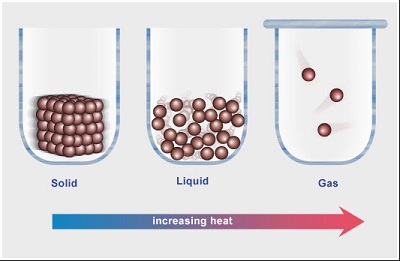
When we begin teaching formal chemistry in high school, we begin, almost as a pedagogical necessity, with some deceptively simple concepts. Humans have been pondering the properties of matter for ages. What IS matter? What is a pure substance? What is a solid? One of the earliest and simplest “definitions” of a solid was anything that could be cut with a knife. Yet science education research studies tell us that some children did not consider either salt or aluminium foil to be solids.
Similarly, how often have you come across advertisements claiming that only their product is ”pure”? How can we decide if the matter on hand is pure or not (and why is purity such an important issue?)? Even a pure substance can look bewilderingly different in different situations – ice and water may not be all that difficult to accept as the same substance; but coal and diamonds?! It was only about four hundred years ago that people were able to entertain such a notion, after Lavoisier spent some of his fortune and heated a diamond, causing it to burn, or combine with oxygen to produce carbon dioxide.
The first CLIx Chemistry module takes up these topics and, using a blend of hands-on activities, discussions and selected simulations that bring alive abstract notions of how particles behave in solids, liquids and gases, intends to enable learners to develop a deeper understanding of such issues. The module should be ready for trials soon after schools re-open post summer vacations.




Comments are closed.